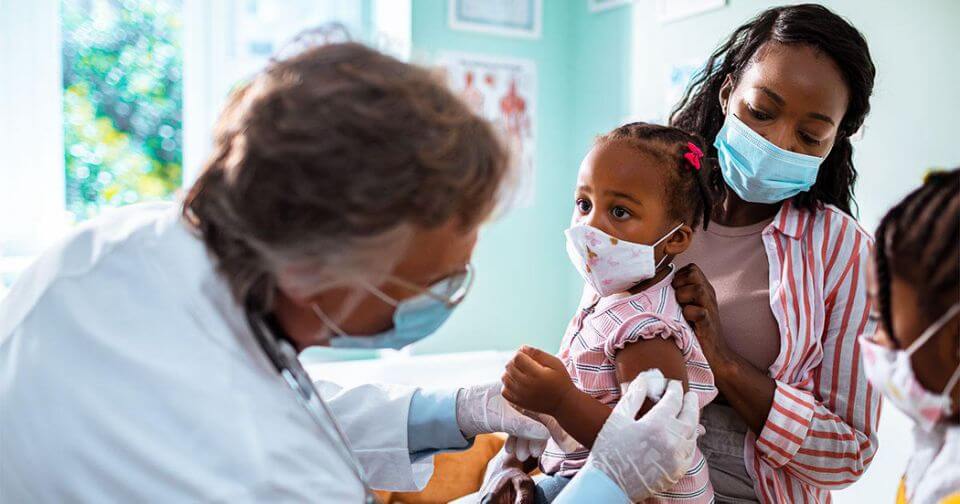
NEW YORK: Moderna has begun testing its COVID-19 vaccine on children and infants between the ages of six months and 12 years old in a study, as the pharmaceutical company seeks to expand approval for its vaccine to children, the company said on Tuesday.
The company said that that the first participants in the KidCOVE study on pediatric COVID-19 vaccinations had been dosed. Moderna is the first of the companies with vaccines authorized in the U.S. to launch testing among infants.
“The first participants have been dosed in the Phase 2/3 study, called the KidCOVE study, of mRNA-1273, the Company’s vaccine candidate against COVID-19, in children ages 6 months to less than 12 years,” the company said.
According to CNN, the clinical trial, called the KidCOVE study, will enroll approximately 6,750 children in the US and Canada between the ages of 6 months and 11 years old.
The trial is divided into two parts: In the first part, different dosages of the vaccine are being tested on the children. Children between the ages of 6 months and 1 year old will receive two doses of the vaccine spaced about 28 days apart at either a 25 or a 50 or a 100 microgram level. Children between the ages of 2 and 11 will receive two doses of the vaccine spaced about 28 days apart at either a 50 or a 100 microgram level.
The findings of part one will be used to determine which dose will be used in part two. For the second part, the trial will expand to include children who are given a saline placebo, which does nothing. The children will be followed for 12 months after their second injection, as per the company’s statement.
Moderna is doing the tests to see if the vaccine protects children from getting sick if they come into contact with coronavirus, according to the clinical trial’s patient information website.
“We are pleased to begin this Phase 2/3 study of mRNA-1273 in healthy children in the U.S. and Canada and we thank NIAID and BARDA for their collaboration,” said Stephane Bancel, Chief Executive Officer of Moderna.
“It is humbling to know that 53 million doses have been administered to people in the U.S. We are encouraged by the primary analysis of the Phase 3 COVE study of mRNA-1273 in adults ages 18 and above and this pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population,” Bancel added
CNN reported that the study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, and the Biomedical Advanced Research and Development Authority at the US Department of Health and Human Services.
Moderna is not the only Covid-19 vaccine currently being tested in children, as the Pfizer/BioNTech Covid-19 vaccine is being studied in children as well. Johnson & Johnson has announced plans to study the vaccine in adolescents, ages 12 to 18.
In December, the US Food and Drug Administration authorized the emergency use of Moderna’s Covid-19 vaccine for adults and of Pfizer/BioNTech’s Covid-19 vaccine for people ages 16 and older.
According to Sputnik, Few children under age 18 have died of COVID-19, less than 300. But the group accounted for more than 10 percent of positive tests for coronavirus infection, according to data from the Centers for Disease Control (CDC) and the American Academy of Pediatrics.
Studies attempting to gauge how readily children spread the virus to adults have yielded conflicting results. In addition, some infected children develop a related disease known as Multisystem Inflammatory Syndrome which typically requires hospitalization, although most victims recover, researchers say. (ANI)